PHOTO
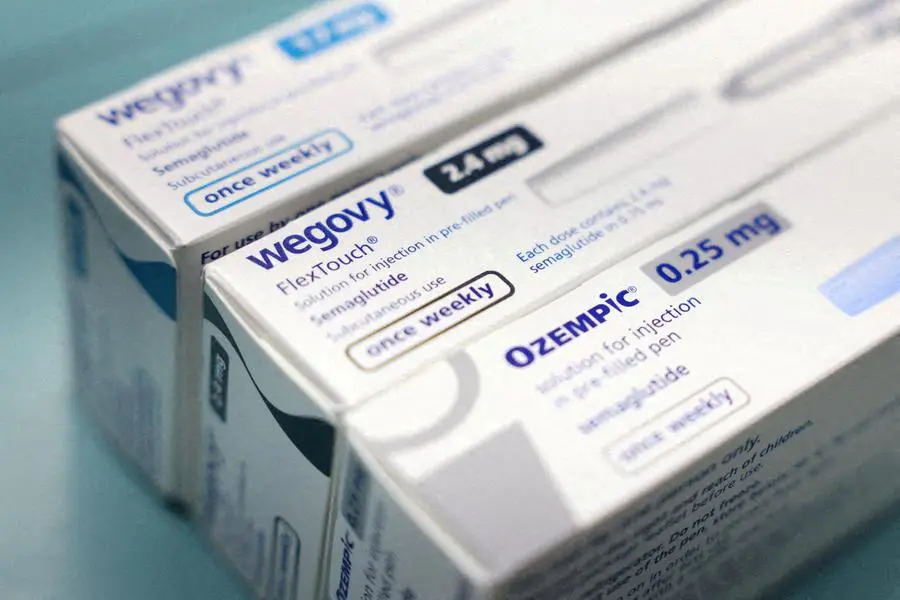
The South African Health Products Regulatory Authority (Sahpra) has expressed concern at the increasing presence of counterfeit Glucagon-like Peptide (GLP-1) products, such as Ozempic, on the local market.
According to Sahpra, GLP-1-containing products, like Ozempic, which help lower blood-sugar levels and promote weight loss, are being made available to the public through websites, social media, and other informal channels.
“These pose a health risk to the public. Sahpra cautions the public to not purchase and/or consume such products as their safety, efficacy, and quality have not been assessed.”
Sahpra has reported an increase in suspected counterfeit Ozempic, chemically known as semaglutide, which is one of the registered products containing GLP-1 agonists from Novo Nordisk.
According to the watchdog, Ozempic in South Africa is registered under two presentations of pre-filled injectable pens, namely 0.25mg and 0.5 mg/dose pen and 1 mg/dose pen.
“Ozempic is registered in South Africa for the treatment of adults with type 2 diabetes to reduce blood-sugar levels for the treatment of adults with insufficiently controlled type 2 diabetes mellitus as an adjunct to diet and exercise.”
Cardiovascular risk reduction
It is also approved to reduce the risk of major cardiovascular events, such as heart attack, stroke, or death, in adults with type 2 diabetes who have known heart disease.
“Ozempic is not registered in South Africa for weight management,” said the authority.
Mounjaro (tirzepatide), developed by Eli Lilly and Company, is also gaining attention in South Africa. It is available in pre-filled pens that come in a single dosage, containing either 2.5mg, 5mg, 7.5mg, 10mg, 12.5mg, or 15mg in a 0.5ml solution for injection.
Mounjaro is indicated for treating type 2 diabetes mellitus but has not yet been imported into and placed on the market in South Africa through Eli Lilly and Company distribution channels.
Just like Ozempic, the watchdog stated that Mounjaro is not approved in South Africa for weight management.
“The complexity of compounding GLP-1 agonists, which are sterile medicines containing complex active substances poses a public-health and safety risk.
“The risk associated with compounded medicines containing GLP-1 agonists are posed by the absence of the evaluation of these medicines by Sahpra and the unknown nature and safety of ingredients used in compounding.”
Unverified compounded products
Compounded products that claim to contain semaglutide have not been verified or evaluated by Sahpra to determine if the active pharmaceutical ingredient is identical to the registered one, as required by the Medicines Act.
“Therefore, it may be substandard and pose a risk to those using them,” said Sahpra, adding that the medicine using an active ingredient that is not contained in a product registered with Sahpra is illegal in terms of the requirements of the Medicines and Related Substances Act.
“The public is urged to purchase only Sahpra-registered products containing GLP-1 agonists, considering these risks.”
According to Section 29 of the Medicines and Related Substances Act, a person who commits an offence will be held criminally liable, and applicable penalties will be enforced.
Sahpra chief ex, Boitumelo Semete-Makokotlela, stressed that safeguarding the wellbeing of the South African public remains a primary concern for the regulatory authority.
“Sahpra is monitoring the supply chain as well as the online platforms for unregistered, sub-standard, and falsified medicines containing or claiming to contain semaglutide. We are also investigating any contraventions relating to the Medicines and Related Substances,” said Semete-Makokotlela.
The public is urged to report any suspected products falsely claiming to be sold as Ozempic or Mounjaro.
Citizens are urged to report through this whistle-blower platform, or Sahpra’s 24-hour hotline 0800 204 307.
All rights reserved. © 2022. Bizcommunity.com Provided by SyndiGate Media Inc. (Syndigate.info).